In 1811, British scientist H1amphrey Davy discovered chlorine dioxide (CIO2). Since its discovery, researchers have never stopped researching chlorine dioxide, including physical, chemical properties, toxicological properties, absorption spectroscopy, and application techniques in various fields. Chlorine dioxide is a strong oxidant. When a typical single electron transfer occurs to form chlorite (CIO2-), its redox potential is 0.95V. When 1 mol of CIO2 is reduced to chloride ion (Cl-), 5 mol electron transfer occurs, so its effective chlorine content is 263% (52.6% × 5), and the oxidation capacity is 2.63 times that of CI2. With its strong oxidizing properties, chlorine dioxide is often used as a disinfectant and is a recognized high-efficiency, broad-spectrum disinfectant that kills almost all microorganisms, including bacterial propagules, bacterial spores, fungi, mycobacteria and viruses. Wait.
Chlorine dioxide is soluble in water and remains in a non-dissociated form in aqueous solution, which can effectively destroy trace organic pollutants in water, such as benzopyrene, hydrazine, chloroform, carbon tetrachloride, phenol, chlorophenol, Cyanide or the like does not cause chlorination reaction when oxidizing organic substances, and does not produce trihalomethanes (THMs) having carcinogenic effects. Therefore, chlorine dioxide has long been used as a disinfectant in a solution state, and is widely used in drinking water treatment. The boiling point of chlorine dioxide is 9.9 ~ 1l ° C, the color of the gas is related to the concentration, yellow-green to orange-red at normal temperature, with good diffusion and penetration characteristics. Utilizing the gaseous state and disinfection characteristics of chlorine dioxide, foreign countries began to study the disinfection technology of gaseous chlorine dioxide in 1999, and obtained many results with ideal results, which made the gas chlorine dioxide disinfection technology develop rapidly. In 2006, relevant research reports began to appear in China, which were used for disinfection of fruits and vegetables, disinfection of space and surface of objects, and removal of formaldehyde pollution. Following the development of foreign technology, there are still some problems and defects in related fields. This paper reviews the research progress of gas chlorine dioxide application technology at home and abroad, and puts forward the problems in the research field, in order to introduce the development status of the technology objectively and quaintly, and to promote the research and application of domestic gas chlorine dioxide application technology. Provide reference.
1 Gas chlorine dioxide space and surface disinfection
Gas disinfectant has good diffusibility and penetrability, and has strong advantages in space and surface disinfection. Due to the complexity of the surface of the object in the space, the traditional disinfection methods such as spraying and wiping no longer meet the requirements of comprehensive disinfection, such as the difficulty of disinfecting the surface of the semi-closed object and the bottom of the device. The commonly used formaldehyde fumigation disinfection has time-consuming and laborious problems, and formaldehyde was clearly listed as a carcinogen by the World Health Organization in 2004. It has been banned in many developed countries such as Europe and the United States, prompting gas chlorine dioxide to enter the historical arena.
In particular, in 2001, in the US anthrax mail incident, the US Environmental Protection Agency (USEPA) evaluated the disinfection effect of gas chlorine dioxide on the entire building, such as the Senate office building suspected of anthrax contamination, and the Brentwood Post Office sorting center in Washington. . The gas chlorine dioxide space disinfection technology has gained wide attention and recognition. In 2006, ClorDiSys's commercial gas chlorine dioxide disinfection machine was approved by USEPA, making the gas chlorine dioxide disinfection machine a commercial promotion road. In 2007, the National Sanitation Foundation (NSF) revised the NSF/ANSI 49 standard to specify that gaseous chlorine dioxide can be used in place of formaldehyde for sterilization of biosafety cabinets. In 2008, Wood and others, with the support of the US Department of Defense, developed a large vehicle-mounted gas chlorine dioxide disinfection system with a target of 9912m. The entire building was sterilized, providing a technical reserve for the overall disinfection of large buildings in the United States in response to ember mail incidents.
In 2015, during the Ebola epidemic in West Africa, American scholars explored the feasibility of gas chlorine dioxide disinfection of EBO virus. Doona et al. applied the portable gas chlorine dioxide sterilizer developed by the Natick Soldier Center in the United States to the sterilization of Ebola-contaminated medical devices. Lwoe and other studies have shown that gaseous chlorine dioxide is not effective in disinfecting the surface of objects containing blood and dirt. The use of gas chlorine dioxide disinfection, the surface, 2003-2016 around the internal and external research and development institutions evaluated the effect of gas chlorine dioxide on the surface of different spaces and objects, some of the main research results are listed in Table 1, indicating gas dioxide Chlorine can completely and quickly disinfect biosafety laboratories and their internal equipment, hospitals, general laboratories, ambulances, libraries, restaurants and other objects.
Table 1 Study results of disinfection of space and surface by gaseous chlorine dioxide
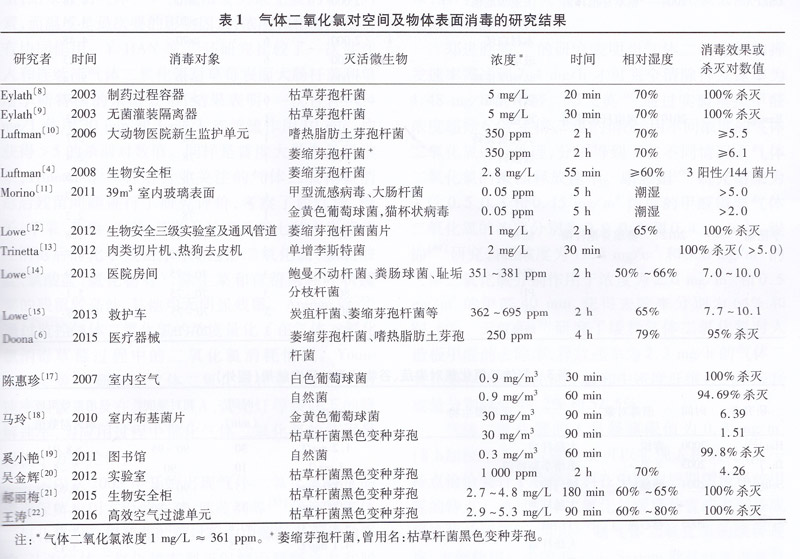
It can be seen from the results of Table 1 that when chlorine dioxide is used for high-level disinfection or sterilization, higher concentration and longer disinfection time are required, and when disinfecting in a general indoor environment such as a library, low use can be used. The concentration of the 8 h weighted concentration value (0.28 mg/m3) was exposed for a long time to achieve the purpose of eliminating common indoor bacteria. In the case of killing bacterial spores, higher relative humidity is required, generally not less than 60% RH. The higher relative humidity can wet the outer wall of the spore to expand it to facilitate the attachment and penetration of gaseous chlorine dioxide, while the high humidity environment increases the risk of corrosion of gaseous chlorine dioxide. Therefore, when gaseous chlorine dioxide is applied to high levels of disinfection or sterilization of space and surface of objects, material compatibility of gaseous chlorine dioxide needs to be considered.
USEPA conducted a systematic experimental study on the compatibility of common electronic equipment and common materials in the laboratory with gaseous chlorine dioxide. The results show that the compatible metals include stainless steel (304SS, 316SS) and hard surface treated with oxidation. Aluminum, etc., commonly used plastics including polyvinyl chloride, polyoxymethylene, polytetrafluoroethylene, etc., provide guidance for the application of gaseous chlorine dioxide.
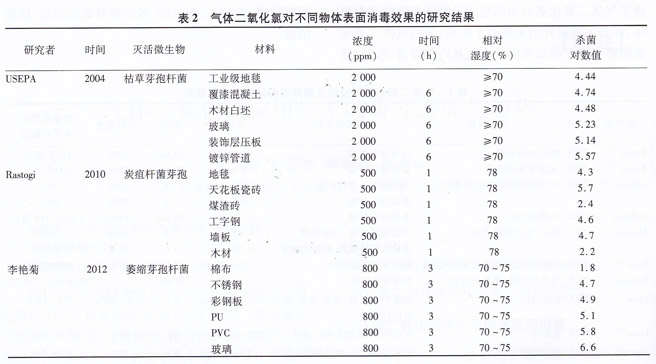
Compared with formaldehyde, ethylene oxide and other gas disinfectants, the disinfection effect of gaseous chlorine dioxide is greatly affected by the surface material properties of the object. Vipin systematically studied the gas chlorine dioxide on various dense and porous buildings in buildings. The killing effect of Bacillus anthracis on the surface of the material; USEPA published a large number of experimental results to illustrate the effect of building materials on the disinfection effect when using gas chlorine dioxide to disinfect buildings; Li Yanju et al. and Wang Yanqiu studied gas chlorine dioxide for biosafety experiments. The killing effect of Bacillus acnes spores on common materials in the room. Some of the results of the above studies are listed in Table 2. The results show that gaseous chlorine dioxide can effectively kill microorganisms on the surface of various materials, but the sterilization effect on complex porous surfaces such as cloth and wood is poor. For stainless steel, glass, plastic, etc. The bactericidal effect of the dense structure surface is also significantly different, and the material surface material of the object needs to be considered in the disinfection application.
2 gas chlorine dioxide fruit and vegetable disinfection
Gas chlorine dioxide can be applied to the surface disinfection of fruits, vegetables, cereals and other foods to prolong the storage period and improve safety. This research field has been receiving continuous attention at home and abroad for a long time, and the disinfection target has been continuously increased, and disinfection power has been carried out. Basic research such as learning models. The Department of Food Science at Purdue University in the United States started the research field with the research team represented by Y.HAN and the subsequent B.s.M.Mallmoud and V.Trinetta teams. A very comprehensive research result has been achieved.
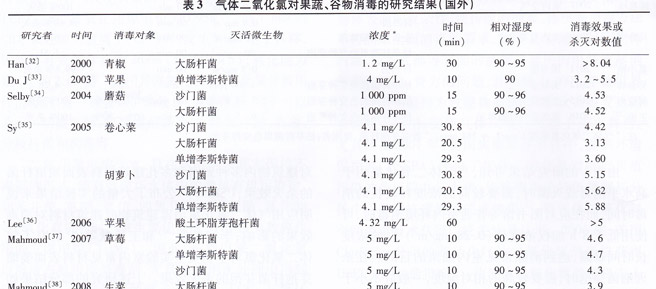
In addition to the evaluation of the disinfection effects listed in Table 3, foreign scholars have also conducted some research on related issues. Y.HAN et al. obtained the response surface model of E. coli on the surface of green pepper treated with chlorine dioxide. The results show that the concentration of chlorine dioxide is the most important factor, while temperature is the most important factor. The concentration and relative humidity have Synergy. Y.HAN et al. also studied the bactericidal effect of single-injection and continuous-flow gas chlorine dioxide on strawberry E. coli and Listeria monocytogenes. The results showed that one-time injection of 4mg/L for 30 min and 3 mg/L continuous The flow was applied for 10 min and a bactericidal logarithm of >5 was obtained. Similarly, the Department of Food Science of Purdue University, Tzinetta et al. conducted research and evaluation on the residual chlorine dioxide disinfection problem of the industry, and investigated the disinfection of seven fruits and vegetables such as tomatoes, oranges, apples, strawberries, lettuce, alfalfa and cantaloupe. After the chemical residue levels (including chlorine dioxide, chlorite, chlorate, chloride, etc.), except for the high residue of lettuce and eucalyptus, there was no significant residue. Arango et al. quantified the consumption of chlorine dioxide in the process of gas chlorine dioxide disinfection of strawberries by monitoring the concentration of gaseous chlorine dioxide. Youn-sukLee et al. studied the diffusion rate factor of gaseous chlorine dioxide in air and the degradation rate under conditions of darkness, UVA, fluorescent lamp, etc., which provided a methodological basis for quantifying the use of gaseous chlorine dioxide in the application process.
Since 2005, the literature on the application of gaseous chlorine dioxide to food disinfection has been published in China. Fu Maorun et al. studied the effect of gaseous chlorine dioxide on the endogenous hormone content during red grape storage. After returning from Purdue University, Hu Shuangqi led a team to study the gas chlorine dioxide on cantaloupe, grape, apple and fresh jujube based on self-developed high-purity chlorine dioxide generator. The bactericidal effect of Salmonella, Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, and saprophytic yeast on the surface of fruits and vegetables, the sterilization rate can reach 99% or more. The gas chlorine dioxide is evaluated for bitter buckwheat, sweet glutinous rice, Oats are lax. The bactericidal effect of Aspergillus flavus and Bacillus subtilis on cereals such as corn indicates that gas chlorine dioxide has a good effect on preventing mildew of cereals, and it is considered that gaseous chlorine dioxide has better effect on smaller volume of grain. The bactericidal effect; the explosion characteristics of gas chlorine dioxide and the law of photodegradation were studied, which provided a scientific basis for the application of gaseous chlorine dioxide in food safety field.
3 gas chlorine dioxide to remove formaldehyde
Decoration pollution directly harms human health, especially when the formaldehyde exceeds the standard and causes leukemia and other accidents. The mainstream news media is more and more concerned by the family and the society. Therefore, the removal of decoration pollution has formed a large-scale industry. In addition to physical methods such as ventilation, ultraviolet irradiation, and activated carbon adsorption, the oxidant can also be reacted with formaldehyde to achieve the purpose of removal. Gas Chlorine Dioxide is a gas at room temperature and has strong oxidizing properties. It reacts with formaldehyde to form formic acid and further deepens the reaction until carbon dioxide and water are formed. Since 2003, domestic researchers have studied the efficiency and influencing factors of gaseous chlorine dioxide removal of formaldehyde.
Zheng Jinsheng and other studies have shown that when the vaporization rate of gaseous chlorine dioxide needs to reach 7.4 mg/h, the formaldehyde with a volatilization rate of 4.48 mg/h can be completely eliminated. Deng Feiying simulated the formaldehyde concentration exceeding 6 times, 4 times and 2 times, and treated with different concentrations of gas chlorine dioxide to obtain the optimal release rate of gaseous chlorine dioxide under different conditions. Kang Zhijuan's research results in the removal of formaldehyde at concentrations of 0.5, 0.3, and 0.15 mg/m3. The concentrations of gaseous chlorine dioxide required to be 0.9, 0.6, and 0.4 mg/m3, respectively, indicate that the concentrations are 12.5 mg/m3 and 5.0 mg/m3. Gas chlorine dioxide was applied to formaldehyde at a concentration of 2.0 mg/m3 and 0.5 mg/m3 for 40 min, respectively, and the removal rates were 64% and 84.6%, respectively. Yunhong studied the removal rate of formaldehyde on wood-based panels by slow-release gas chlorine dioxide. The release rate of 2.3 mg/h gas chlorine dioxide for 9 days reduced the formaldehyde emission of particleboard and medium density fiberboard by 49.2% and 52.5 respectively. %.
The personnel exposure limit of gaseous chlorine dioxide is 0.3 mg/m3 (8h weighted concentration), which means that man-machine coexistence can be achieved. This feature is precisely the characteristics of the slow release of formaldehyde in the middle and late stages of the decoration materials. The sustained release rate of gaseous chlorine dioxide can be controlled by a gas chlorine dioxide sustained release device or a gel-formed sustained release agent, which is convenient to use. United States. The Biockle System commercial indoor air purification box uses a gas chlorine dioxide slow release gel for the removal of indoor formaldehyde and organic substances such as benzene, which is popular in China.
4 problems
Domestic research on gas chlorine dioxide has accumulated over the past decade and has achieved many research results, basically following the technical level of European and American countries, but there are also some problems.
4.1 Research team is less
The domestic gas chlorine dioxide researchers mainly include the team represented by Hu Shuangqi and Jin Riya from North University of China and the team represented by Qi Jiancheng and Wu Jinhui. North University of China focuses on the food safety field, while the Academy of Military Medical Sciences focuses on the field of biosafety, and has not formed more research teams that can focus on results. Can form research hotspots.
4.2 Less basic research
Domestic research results mainly focus on applied technology research, and the mouth is evaluated for the disinfection effect of different subjects, but lacks relevant basic research.
Such as the mechanism of gas chlorine dioxide disinfection; the kinetic model of the reactor for preparing gas chlorine dioxide by gas-solid reaction, the method for detecting the life of the reactor; the quality variation of the chlorine dioxide in the disinfection of fruits and vegetables and grains, disinfection by-products and Its residue; the influence of organic matter and covering on the surface of the object on the disinfection effect.
4.3 less equipment support
The two teams of North University and the Academy of Military Medical Sciences have self-developed gas chlorine dioxide generating equipment. In comparison, North Central University is a liquid-liquid reaction type, and the generated gas contains a small amount of chlorine gas components; the Academy of Military Medical Sciences It is a gas-solid reaction type, and the generated gas is pure chlorine dioxide except nitrogen. Both have their own advantages and disadvantages, but none of them have formed commercial products for promotion. In addition, there is a lack of accurate concentration on-line detection devices when conducting application studies at very low concentrations (<o.28 mg/m3). In a disinfection application scenario where it is not suitable to install a concentration online monitoring device, a reliable chemical indicator card will be very valuable. The CD-CHECK of ClorDisys in the United States is such a product, and there is still no relevant research report in China. .
4.4 The absence of national standards in the five national standards for chlorine dioxide that have been promulgated and implemented are related to chlorine dioxide solution rather than gaseous chlorine dioxide, including GB 26366-2010 “Sanitary Standards for Chlorine Dioxide Disinfectants”. Emphasize the application of the solution form. Gas chlorine dioxide, as another form of chlorine dioxide disinfectant, has demonstrated superiority and applicability and should be included in relevant standards or draft national standards for the application of gaseous chlorine dioxide in various fields.
5 development trend
Through the analysis of domestic and foreign literatures, gas chlorine dioxide has more research support in the fields of space and surface disinfection, food disinfection, and formaldehyde removal. It has its superiority and has a good promotion prospect. Especially in the disinfection of space and surface of objects, it is easier to promote because it does not involve sensitive issues such as food safety and health hazards.
In the field of biosafety, the competitive technology of gaseous chlorine dioxide is vaporized hydrogen peroxide, both of which have their own advantages and disadvantages. The gaseous chlorine dioxide is prepared on-site as a chemical dangerous substance, while vaporized hydrogen peroxide cannot penetrate high-efficiency air filtration. Therefore, there are occasions for each.
Among them, gaseous chlorine dioxide is more suitable for the overall disinfection of the laboratory, including laboratory ventilation ducts and laboratory internal equipment.
Another important application scenario for gaseous chlorine dioxide is the overall disinfection of biopharmaceutical plants. As some of the global biopharmaceutical companies' industrial chains are transferred from developed countries and regions such as Europe, the United States and Japan to China, India and other developing countries, as well as China's support for the bio-pharmaceutical sector, large-scale biopharmaceutical cleanrooms are springing up. Responsible companies have largely abandoned the formaldehyde fumigation method, and the same as the gas chlorine dioxide competition is vaporized hydrogen peroxide. As described above, the gas chlorine dioxide is more suitable for one-time overall disinfection of extremely large spaces, including There is a huge market prospect for sterilization before production and sterilization between product conversions.
Because gas chlorine dioxide has good diffusion characteristics and material compatibility, it is suitable for the disinfection of precision equipment containing electronic components, optical components and complex structures. With the upgrading of information technology, more and more precision equipment is put into the military, gas Chlorine dioxide will make a difference in the elimination of contaminated biological warfare agents in precision equipment.
In the field of health care, gaseous chlorine dioxide should be further promoted. First of all, gaseous chlorine dioxide can be used for disinfection of special parts such as infectious wards, burn wards, clean surgical departments, etc. Secondly, very low concentration of chlorine dioxide can also be used for human-computer co-existing air purification in general clinics and wards; again, gas Chlorine dioxide can be used for disinfection or sterilization of medical devices. For example, the Academy of Military Medical Sciences applies gas chlorine dioxide to high-level disinfection of digestive endoscopes. US Doona uses gas chlorine dioxide for portable sterilization of surgical instruments. .
Gas Chlorine Dioxide is a safe, broad-spectrum and highly efficient gas disinfectant. It is believed that with more researchers and investors, it will be more widely recognized and further improve the technical level of disinfection in China. .





